CASE REPORT | https://doi.org/10.5005/jp-journals-10024-2731 |
A Case Report of Maxillary Aspergillosis with Unusual Clinical and Imaging Presentations
1Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia
2Department of Oral Diagnostic Sciences, Faculty of Dentistry, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia
Corresponding Author: Fatima M Jadu, Department of Oral Diagnostic Sciences, Faculty of Dentistry, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia, Phone: +966-254-0000, e-mail: fjadu@kau.edu.sa
How to cite this article Jan AM, Jadu FM. A Case Report of Maxillary Aspergillosis with Unusual Clinical and Imaging Presentations. J Contemp Dent Pract 2020;21(2):211–214.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Aim: The aim of the present study was to present a case of maxillary aspergillosis with unusual clinical presentation and imaging findings.
Background: The range of lesions and conditions that affect the maxillary sinus is wide and vast. This necessitates a dynamic multidisciplinary approach for proper diagnosis and effective management.
Case description: We present an unusual case of maxillary aspergillosis that mimicked apical periodontitis and was most consistent with lymphoma from the diagnostic imaging perspective. Histopathology, however, established the correct diagnosis; appropriate management commenced promptly.
Conclusion: It is imperative that maxillary aspergillosis be diagnosed and managed properly and promptly to improve prognosis. Advanced imaging is required, but it plays an adjunct role to histopathology. Long-term follow-up is also necessary to ensure complete resolution of the infection.
Clinical significance: Correlating clinical and imaging findings is a crucial step and any inconsistencies should be resolved promptly so as not to delay adequate management. Histopathology often serves to dispute any inconsistencies and allows the establishment of a proper diagnosis.
Keywords: Aspergillosis, Fungal, Infection, Maxilla.
INTRODUCTION
Lesions of the maxillary sinuses are versatile due to the numerous tissues in the area. This is further complicated by the proximity of the maxillary sinus to the alveolar process and dentition.1 Odontogenic lesions often present with symptoms related to the maxillary sinus and the opposite is correct as well.1 Therefore, dentists must be well versed in all conditions of the maxillary sinus and must be aware of how to differentiate the various disorders to provide the best patient care.2 Reaching the proper diagnosis is the first step and this usually requires imaging; however, imaging alone is not sufficient. The diagnostic process is a vital dynamic process that requires effective communication and efficient collaboration.
CASE DESCRIPTION
Clinical Presentation
A 39-year-old patient presented to an academic oral and maxillofacial surgery service complaining of pain related to the left posterior maxillary area. The symptoms were most consistent with apical periodontitis of teeth #25 and #27, especially considering the presence of defective restoration on the tooth #27. All maxillary left posterior teeth were tested with Endo-Ice® and all were vital. No gingival recession or mobility were noted for any of the maxillary left posterior tooth. However, teeth #25 and #27 were tender to palpation and tenderness was more pronounced buccally. The medical, family, and occupational histories did not reveal any significant findings; and there were no known drug allergies. Extraoral examination revealed minor facial swelling over the left cheek (Fig. 1). Upon intraoral examination, a vestibular submucosal swelling (Fig. 1) was evident spanning from the apical area of the maxillary left second premolar to the apical area of the second molar in the same quadrant (approximately 12 × 15 mm). No erythema was noted. The swelling was not excreting any material through the cervices of the teeth; and there was no sinus tract formation. The differential diagnosis included residual cyst, osteomyelitis, and peripheral odontogenic tumor.
Imaging Examination and Interpretation
The imaging examination began with a panoramic radiograph (Fig. 2). This radiograph demonstrated a loss of the cortical boundaries of the left maxillary sinus: The lateral and medial cortices and the floor of the sinus were all missing. Opacification of the same sinus was also noted. This prompted a cone-beam computed tomography (CBCT) request. The CBCT images demonstrated mucosal thickening in both maxillary sinus, which was more pronounced in the left sinus. The most striking feature was a perceived mass effect of what appeared to be a relatively well-defined soft tissue mass causing bowing and discontinuation of the anterior wall of the left maxillary sinus. A representative CBCT image is presented in Figure 3. The diagnostic images were most consistent with lymphoma although osteomyelitis and granulomatosis were also considered.

Figs 1A and B: (A) Extraoral picture; (B) Intraoral picture demonstrating the left midface swelling and the corresponding vestibular swelling (white arrow)

Fig. 2: Panoramic radiographs demonstrating loss of the cortical boundaries of the left maxillary sinus (red arrows)
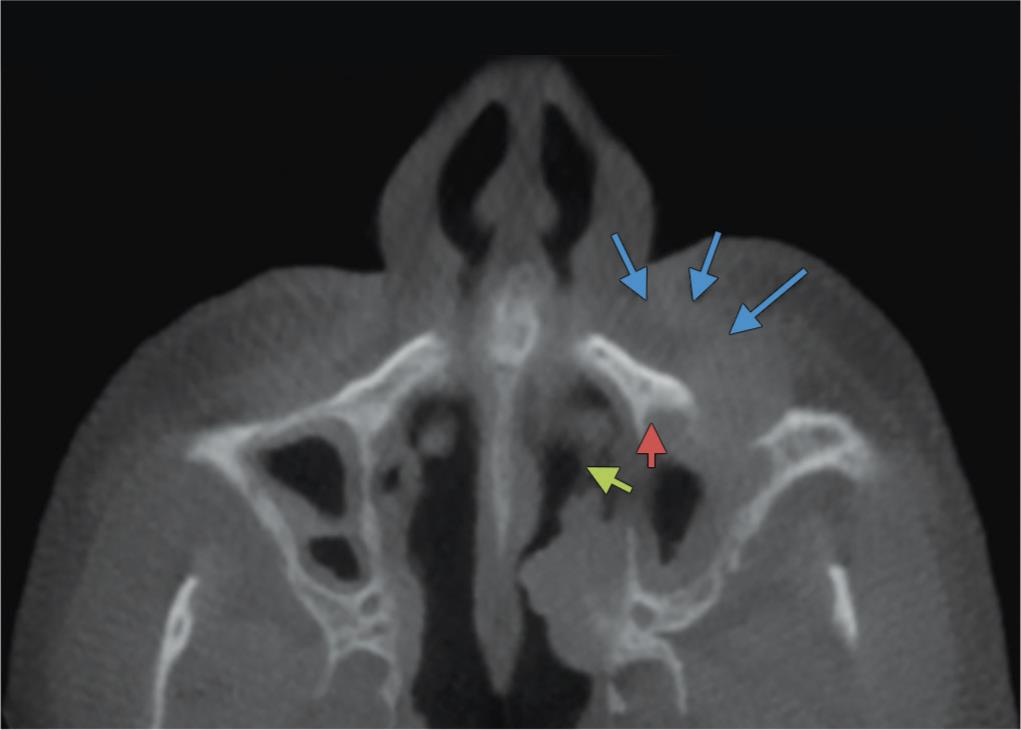
Fig. 3: Axial reformatted cone-beam computed tomography image demonstrating the perceived soft tissue mass (blue arrows) causing bowing and discontinuation of the anterior wall of the left maxillary sinus (red arrow). We also note a discontinuation of the medial wall of the left maxillary sinus (green arrow)

Fig. 4: Photomicrograph of hematoxylin and eosin (magnification ×400) stained section showing the engorged blood capillaries and the multinucleated giant cells (red arrow)
Surgical Management
The clinical and radiographic findings suggested that an excisional biopsy should be performed. An incisional biopsy was dismissed to ensure that an adequate tissue sample is available for histopathological examination. The head and neck areas were prepped and draped in the customary fashion. Local anesthesia was achieved with 4% articaine with 1:100 k epinephrine using 10.20 mL for left posterior superior alveolar nerve block, middle superior alveolar nerve block, and greater palatine block. A partial thickness mucoperiosteal flap was raised by extending 1.5 cm anterior and posterior to the confines of the submucosal mass. The subperiosteal dissection was done 2 mm beyond the confines of the swelling followed by subperiosteal dissection of the mass of the maxillary bone. The anterior wall of the maxillary sinus was found to have a 5 × 5 mm dehiscence, but the sinus membrane was intact. Irrigation with physiological saline followed, and the surgical specimen was submitted for histopathological examination. The patient was put on a 1-week course of oral amoxicillin 500 mg three times daily, ibuprofen 600 mg 4 times daily, and a chlorhexidine mouthrinse.
Histopathologic Findings and Diagnosis
The specimen was examined via hematoxylin and eosin stained sections. These slides revealed engorged capillaries with red blood cells and a variety of inflammatory cells, including lymphocytes, plasma cells, and eosinophils. Numerous multinucleated giant cells were detected—some engulfing spores (Fig. 4). Areas of necrosis were also evident. This prompted a request for periodic acid–Schiff, Gomori methenamine silver (GMS), and Ziehl–Neelsen (ZN) staining. Periodic acid–Schiff (PAS) and GMS stains were positive while ZN staining was negative. Gomori methenamine silver staining specifically demonstrated grayish-black branching septate hyphae at 45° consistent with aspergillosis species (Fig. 5). The final diagnosis was aspergillosis and was confirmed by three certified oral and maxillofacial pathologists.
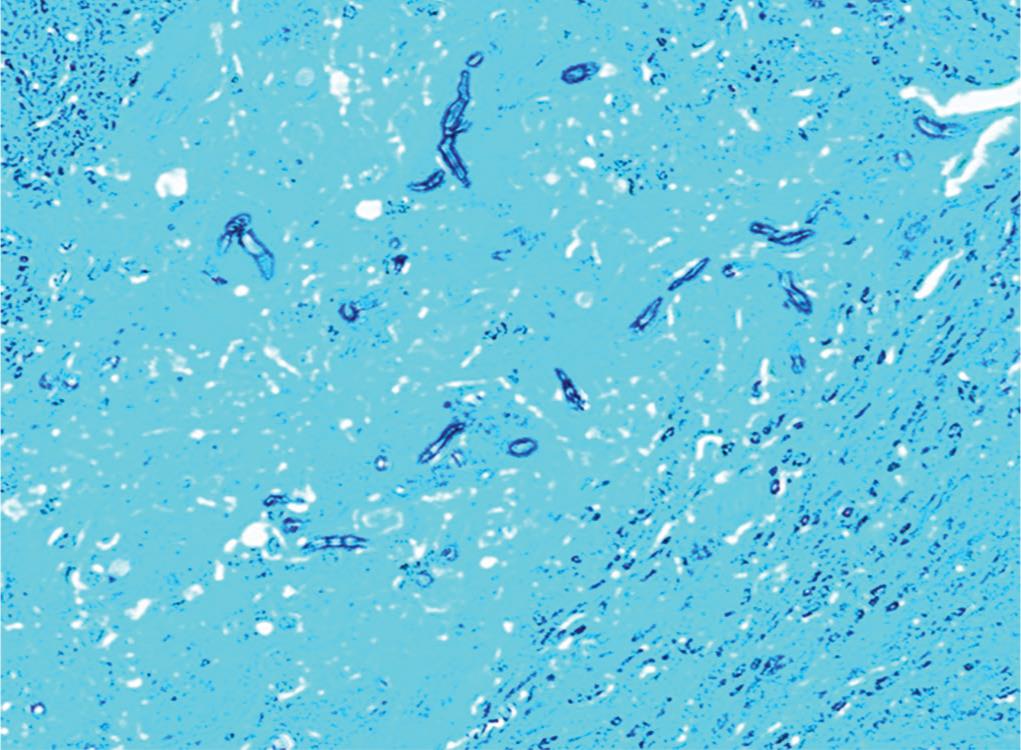
Fig. 5: Photomicrograph of Gomori methenamine silver (magnification ×200) stained section demonstrating the characteristic grayish black branching septate hyphae of aspergillosis

Fig. 6: Axial reformatted cone-beam computed tomography image at 1 year follow-up demonstrating reestablishment of the cortical boundaries of the left maxillary sinus. The anterior and postrolateral walls of the left maxillary sinus appear thick due to the healing process. Some mucosal thickening still remains
Medical Management
After histopathology confirmed the diagnosis of aspergillosis, the patient was promptly referred to the infectious disease service in the same academic institution. An endoscopy of the paranasal sinuses was requested and performed by an ear, nose, and throat specialist. During the procedure, a specimen was collected from the left maxillary sinus and submitted for microbial culture that confirmed the diagnosis of aspergillosis and determined the offensive species to be Aspergillus fumigatus. The results of the endoscopy confirmed localization of the infection to the left maxillary sinus without the involvement of the ethmoidal, sphenoidal, or frontal sinuses. No predisposing factors, such as uncontrolled diabetes mellitus, trauma, or medication use, or misuse were confirmed in this case, which makes this case unique in presentation. The patient was managed with voriconazole 200 g two times daily for 3 months. For the first 2 weeks, the medication was administered intravenously, and the patient was followed up on a daily basis. Then, for the remainder of the treatment period, the patient was followed biweekly and the medication was given orally. This resulted in complete resolution of the infection without any further surgical intervention. The patient was symptom free with no signs of residual infection or recurrence at the 1-year follow-up appointment; and this was confirmed both clinically and with imaging. The 1-year follow-up CBCT images revealed reestablishment of the cortical boundaries of the left maxillary sinus. In addition, the anterior and posterolateral walls of the left maxillary sinus appeared thick due to the healing process. Some mucosal thickening was still evident. These imaging findings (Fig. 6) are clear indications of the resolution of the infection and healing.
DISCUSSION
Aspergillus is a fungus commonly found in humid areas and damp growing on decaying or decomposing matter.3 The spores of this fungus are commonly inhaled and are usually found in the upper respiratory tract and lungs of primarily immunocompromised subjects.3 There are reports, however, of the fungus causing destructive lesions in the paranasal sinuses of healthy adults.3 The maxillary sinuses are the most commonly affected of all the paranasal sinuses.4,5 Introduction into the maxillary antrum can sometimes occur at the time of a dental procedure such as extraction, root canal therapy, or placement of a dental implant.4,5 The first report of aspergillosis in the maxillary sinus was made by Zarniko in 1891.3
The mechanism by which sinus fungal infections cause destructive lesions are believed to be the induction of tissue necrosis.3 Several predisposing factors have been suggested, including uncontrolled diabetes mellitus, inappropriate use of antibiotics, trauma, radiation therapy, and corticosteroid use.4 Clinical manifestations are usually ambiguous and range from nasal congestion and discharge to pain and headaches.5 The prognosis of maxillary aspergillosis is poor if not managed appropriately and promptly.4 The mortality rate increases in immunocompromised patients.4
Diagnosis is usually established through histopathology and microbiology. Histopathologic examinations are often specific enough to yield an accurate diagnosis on which treatment can be based.6 However, microbial cultures of aspergillosis are necessary for confirming the diagnosis and determining the species.7 In this case report, the diagnosis was determined by histopathological examination using special staining with PAS, GMS, and ZN. In addition, the results of the microbial culture that was obtained during the endoscopy procedure further confirmed the diagnosis and established the species causing the infection to be A. fumigatus.
The imaging features of maxillary aspergillosis are nonspecific, but usually unilateral.5 Advanced imaging in the form of CT or magnetic resonance imaging is necessary. CBCT can also be used to assess the osseous structures of and around the maxillary sinus. The differential diagnosis for such destructive lesions usually includes neoplasms, such as squamous cell carcinoma and lymphoma.4,5 Other conditions to consider are sinusitis, tuberculosis, sarcoidosis, and Wegner’s granulomatosis.4 The diagnosis of maxillary aspergillosis is primarily based on histopathology and microbiology; imaging can offer more details on the extent of disease.
Aspergillosis is a serious life-threatening condition and can be fatal if left untreated.8,9 Although the diagnosis of osteomyelitis was not established here, it has been reported in one case that was also successfully treated with variconazole,10 which is a broad-spectrum triazole antifungal agent. It inhibits the fungal cytochrome P-450 and sterol C-14 α-demethylation. It also decreases the formation of ergosterol and inhibits fungal cell membrane formation.11 Variconazole is considered the first line of therapy for patients with invasive aspergillosis.12,13 It is also proven to be effective in treating fungal infections in patients with solid pulmonary tumors.14 Adjusting the variconazole dose can be challenging, especially in patients with compromised liver function and patients with hematopoietic cell transplant.15,16 Fortunately, in this case report, the patient was free of any predisposing factors affecting the immune system.
Deep fungal infections of the maxilla are fortunately rarely encountered. These infections usually affect all the paranasal sinuses in an aggressive fashion. Hence, they must be treated promptly and appropriately to prevent fatal complications, such as spread to the cranial base. The treatment options commonly include surgical resection of infected tissues especially if the diagnosis of fungal osteomyelitis is also made. In one case report, a patient with type 2 diabetes mellitus underwent extraction of multiple teeth after which diffuse swelling of the midfacial region developed the diagnosis of osteomyelitis was made.17 Histopathology and microbiology further ascertained the presence of mucormycosis and aspergillosis.17 This patient was treated with sequestrectomy and saucerization under general anesthesia.17 This was followed with antifungal treatment with IV amphotericin B (1 mg per kg per day) and oral variconazole (200 mg two days daily) for 12 weeks while monitoring liver and kidney functions, blood sugar levels, and complete blood count.17 In this particular case, amphotericin B resulted in neutrophilic leukocytosis.17 Therefore, amphotericin B was discontinued.17 Then, after confirmation of the resolution of the fungal infection, a permanent obturator was constructed and postoperative healing was reported to be satisfactory.17
Invasive aspergillosis is a common complication in patients with hematologic malignancies and it increases the mortality rates in this patient population.18 This is especially true for patients undergoing new targeted biological and cellular therapies.18 To further complicate matters, antifungals commonly inhibit cytochrome enzymes that modulate certain kinase inhibitors used for the management of the underlying malignant conditions.18 This makes the management of such patients and the choice of medication and dosage challenging.18,19
CONCLUSION
It is imperative that maxillary aspergillosis be diagnosed and managed properly and promptly to improve prognosis. Advanced imaging is required; however, it plays an adjunct role to histopathology and microbiology. Long-term follow-up is also necessary to ensure complete resolution of the infection.
CLINICAL SIGNIFICANCE
Correlating clinical and imaging findings is a crucial step and any inconsistencies should be resolved promptly so as not to delay adequate management. Histopathology often serves to dispute any inconsistencies and allows the establishment of a proper diagnosis.
REFERENCES
1. Sheethal HS, Rao K, Umadevi HS, et al. Odontogenic keratocyst arising in the maxillary sinus: a rare case report. J Oral Maxillofac Pathol 2019;23 (Suppl. 1):74–77.
2. Bell GW, Joshi BB, Macleod RI. Maxillary sinus disease: diagnosis and treatment. Br Dent J 2011;210(3):113–118. DOI: 10.1038/sj.bdj.2011.47.
3. Tamgadge AP, Mengi R, Tamgadge S, et al. Chronic invasive aspergillosis of paranasal sinuses: a case report with review of literature. J Oral Maxillofac Pathol 2012;16(3):460–464. DOI: 10.4103/0973-029X.102522.
4. Peral-Cagigal B, Redondo-Gonzalez LM, Verrier-Hernandez A. Invasive maxillary sinus aspergillosis: a case report successfully treated with voriconazole and surgical debridement. J Clin Exp Dent 2014;6(4):e448–e451. DOI: 10.4317/jced.51571.
5. Torul D, Yuceer E, Sumer M, et al. Maxillary sinus aspergilloma of odontogenic origin: report of 2 cases with cone-beam computed tomographic findings and review of the literature. Imaging Sci Dent 2018;48(2):139–145. DOI: 10.5624/isd.2018.48.2.139.
6. Ghosh A, Magar DG, Thapa S, et al. Histopathology of important fungal infections—a summary. J Clin Pathol Nepal 2019;9:1490–1496. DOI: 10.3126/jpn.v9i1.23377.
7. Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev 2011;24(2):247–280. DOI: 10.1128/CMR.00053-10.
8. Chermetz M, Gobbo M, Rupel K, et al. Combined orofacial aspergillosis and mucormycosis: Fatal complication of a recurrent paediatric Glioma—case report and review of literature. Mycopathologia 2016;181(9–10):723–733. DOI: 10.1007/s11046-016-0021-8.
9. Drage N, Rogers S, Greenall C, et al. Incidental findings on cone beam computed tomography in orthodontic patients. J Orthod 2013;40(1):29–37. DOI: 10.1179/1465313312Y.0000000027.
10. Bathoorn E, Escobar Salazar N, Sepehrkhouy S, et al. Involvement of the opportunistic pathogen Aspergillus tubingensis in osteomyelitis of the maxillary bone: a case report. BMC Infect Dis 2013;13:59. DOI: 10.1186/1471-2334-13-59.
11. Gunbatar H, Demir C, Kara E, et al. Successful management of pulmonary hemorrhage and aspergillosis in a patient with acute myeloid leukemia (AML-M3). Respir Med Case Rep 2015;16:65–68. DOI: 10.1016/j.rmcr.2015.07.003.
12. Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 2002;347(6):408–415. DOI: 10.1056/NEJMoa020191.
13. Herbrecht R. Voriconazole: therapeutic review of a new azole antifungal. Expert Rev Anti Infect Ther 2004;2(4):485–497. DOI: 10.1586/14787210.2.4.485.
14. Dib RW, Khalil MC, Fares J, et al. Invasive pulmonary aspergillosis: Comparative analysis in cancer patients with underlying haematologic malignancies versus solid tumors. J Hosp Infect 2019. DOI: 10.1016/j.jhin.2019.09.020.
15. Patel JN, Hamadeh IS, Robinson M, et al. Evaluation of CYP2C19 genotype-guided voriconazole prophylaxis after allogeneic hematopoietic cell transplant. Clin Pharmacol Ther 2019. DOI: 10.1002/cpt.1642.
16. Ren QX, Li LX, Mu JS, et al. Population pharmacokinetics of voriconazole and optimization of dosage regimens based on monte Carlo Simulation in liver cirrhosis patients. J Pharm Sci 2019;108(12):3923–3931. DOI: 10.1016/j.xphs.2019.09.019.
17. Srivastava A, Mohpatra M, Mahapatra A. Maxillary fungal osteomyelitis: a review of literature and report of a rare case. Ann Maxillofac Surg 2019;9(1):168–173. DOI: 10.4103/ams.ams_218_18.
18. Girmenia C. New hematologic populations at risk of invasive aspergillosis: focus on new targeted, biological, and cellular therapies. F1000Res 2019;8:1–9. DOI: 10.12688/f1000research.17836.1.
19. Dominic A, Sekar B, Murali S. Invasive aspergillosis of the maxilla—an unusual report. J Int Oral Health 2012;4(2):47–52.
________________________
© The Author(s). 2020 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.