ORIGINAL RESEARCH | https://doi.org/10.5005/jp-journals-10024-3104 |
Connective Tissue Graft vs Platelet-rich Fibrin in the Treatment of Gingival Recessions: A Randomized Split-mouth Case Series
1–3Department of Periodontology, Pontificia Universidad Católica Madre y Maestra, Santo Domingo, Dominican Republic
4Department of Periodontology, Universidad del Desarrollo, Santiago, Chile
5Department of Family, Population and Preventive Medicine, Stony Brook University, New York, New York, United States
6Department of Periodontology, Stony Brook University, New York, New York, United States
Corresponding Author: James R Collins, Department of Periodontology, Pontificia Universidad Católica Madre y Maestra, Santo Domingo, Dominican Republic, Phone: +1-809-535-0111, Ext. 3291 e-mail: jamescollins@pucmm.edu.do
How to cite this article: Collins JR, Cruz A, Concepción E, et al. Connective Tissue Graft vs Platelet-rich Fibrin in the Treatment of Gingival Recessions: A Randomized Split-mouth Case Series. J Contemp Dent Pract 2021;22(4):327–334.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Aim and objective: Coronally advanced flap (CAF) with connective tissue graft (CTG) has been considered the gold standard for obtaining complete root coverage. However, some limitations have been reported with the use of CTG, especially because it increases morbidity and leads to postoperative pain and bleeding. Recently, platelet-rich fibrin (PRF) has been used in periodontal plastic surgery for the treatment of gingival recessions (GRs). The aim of this study was to evaluate the outcome of PRF combined with a CAF (test) compared to de-epithelialized connective tissue graft (DeCTG) + CAF (control) for GR coverage.
Materials and methods: Ten healthy patients exhibiting mandibular or maxillary Miller class I and II were treated with PRF + CAF or DeCTG + CAF. GR, probing depth (PD), and gingival thickness (GT) were evaluated at baseline, 6 weeks, and 28 weeks postoperatively.
Results: GR, PD, and GT differences between the test and control groups at 28 weeks were not statistically significant. GR was 3.30 ± 1.25 mm and 3.00 ± 1.63 mm (control vs test) group (baseline) and −0.10 ± 0.32 vs −0.20 ± 0.42 mm (7 months), respectively.
Conclusion: Within the limitations of the present study, it can be concluded that localized gingival recessions could be successfully treated with CAF + PRF or CAF + DeCTG.
Clinical significance: This study suggests that PRF membrane may be an alternative and valid graft material for treating localized gingival recessions Miller class I and II.
Keywords: Connective tissue graft, Gingival recession, Platelet Rich Fibrin, Root coverage, Randomized Clinical Trial.
INTRODUCTION
Gingival recession (GR) is defined as the exposure of the tooth root surface due to the migration of the apical gingival margin tissue relative to the cementoenamel junction (CEJ).1 GR is currently a pathological condition that has high prevalence rates; indistinctly occurs in young and adults; its etiology is multifactorial, among these factors are anatomical factors, accumulations of biofilm due to lack of keratinized tissue, incorrect brushing technique (including excessive brushing), occlusal trauma, placement of restoration margins subgingivally and orthodontic treatment.2,3
To cover the exposed root surface, several periodontal plastic surgical techniques have been described over the last decades including the free gingival graft, the coronally advanced flap (CAF), the coronally advanced combined with connective tissue graft (CTG), and various regenerative procedures, such as the use of resorbable and nonresorbable barriers, enamel matrix derivative or the application of platelet-rich plasma (PRP) in combination with CAF.4-9 Apart from the traditional techniques to harvest CTGs to improve gingival thickness (GT) or cover exposed roots, the de-epithelialized connective tissue grafts (DeCTGs) have been described as an alternative method.10
CAF combined with a CTG has been referred to as the gold standard in root coverage.11,12 On the other hand, autologous platelet concentrates, like platelet-rich fibrin (PRF) and PRP, are a great alternative to CTG in the treatment of GR reducing morbidity and discomfort. PRF is an autologous platelet and leukocyte concentrate biomaterial obtained by a safe and simple procedure. Also, PRF does not require biochemical blood handling; its three-dimensional fibrin network promotes effective neovascularization, accelerated wound closure, and fast cicatricial tissue remodeling and has been used in many fields of regenerative medicine in humans for its healing properties.13
The aim of the present study was to evaluate the clinical effectiveness of CAF + PRF or CAF + CTG for the treatment of localized Miller class I and II GR.
MATERIALS AND METHODS
The study was designed as a split-mouth, randomized, clinical trial on the treatment of a single GR. Two different treatment modalities were assessed: the CAF with a DeCTG (DeCTG + CAF; control group) was compared to the CAF with a PRF membrane (CAF + PRF; test group) in terms of clinical outcomes and postoperative morbidity. Study candidates reported to the Department of Periodontology at the Pontificia Universidad Católica Madre y Maestra, Santo Domingo, Dominican Republic. Potential subjects, who have completed the medical and dental history, were screened by the dental examiner. The initial screening procedure consisted of a soft tissue assessment before proceeding with study eligibility evaluations.
Ethical Considerations
Informed consent was obtained from all patients. All good clinical practice standards were applied and maintained throughout the study. The protocol used for this study is in accordance with the Declaration of Helsinki and was reviewed and approved (COBE-FACS-EST-CSTA-002-1-2015-2016) by the Institutional Review Boards, Bioethics Committee (COBE) Faculty of Health Sciences, Pontificia Universidad Católica Madre y Maestra, Santo Domingo, Dominican Republic.
Participants Selection
Eligible patients were registered for an initial visit. Patients were included, if they (1) were aged 18 years and older, systemically and periodontally healthy; (2) were nonsmokers; (3) presented of similar bilateral or contralateral Miller class I or II recessions at maxillary or mandibular incisors, canine, or first premolars with aesthetic compromise or thermal sensitivity; (4) periodontal probing depths (PDs) ≤2 mm and areas of keratinized gingiva width ≥2 mm, with no carious cervical lesions >0.5 mm; (5) 3 mm or more of recession depths (6) not taking any medication that could affect the tissue response and remodeling; (7) absence of restorations and tooth vitality in the area to be treated; (8) thickness of ≥2.5 mm in the palatal donor site. Patients were excluded if they were (1) pregnant women; (2) those who had taken antibiotics and/or anti-inflammatory drugs in the previous month before surgery; (3) presence of active periodontal disease; (4) previous surgical attempt to correct GR; (5) inability or unwillingness to complete the trial and refuse to sign the informed consent form. The first 10 consecutive patients, who completed all the inclusion criteria, were invited to participate (mean age 45.6 ± 11.4, 30–62 years, five men, five women).
The study protocol involved a screening appointment to verify eligibility, followed by initial nonsurgical periodontal therapy to establish optimal plaque control and gingival health conditions. Postoperative clinical evaluation was performed 1, 6, and 28 weeks after surgery (Flowchart 1).

Flowchart 1: Flow diagram of patients in the study and study design (CAF, coronally advanced flap; PRF, platelet rich fibrin; DeCTG, de-epithelialized connective tissue graft)
Randomization and Surgical Procedure
Following baseline examination, all participants received a session of prophylaxis including oral hygiene instructions, scaling, and professional tooth cleaning with the use of a rubber cup and a low-abrasive polishing paste. Surgical treatment of the recession defects was not scheduled until the patient could demonstrate an adequate standard of supragingival plaque control.
The bilateral split-mouth localized gingival recession defects selected for treatment were randomly assigned to receive CAF + PRF (test group) or CAF + DeCTG (control group) using a coin randomization method, immediately before the surgery. The assignment was performed by a different examiner (AC). Participants and the surgeon were blinded in all the stages of the procedure. All surgical procedures were performed by the same surgeon (JC).
Surgical Protocol
Following local anesthesia, the exposed root surfaces were polished with a rubber cup and pumice powder prior to flap elevation (Fig. 1A). Local anesthesia was administered in the recipient site and palate area using 2% lidocaine with 1:100,000 epinephrine (DFL, Rio de Janeiro, Brazil). Control group: a horizontal incision was made with a scalpel to design an envelope flap. The horizontal incision of the envelope flap consisted of oblique submarginal incisions in the interdental areas, incisions that continued with the intrasulcular incision at the recession defect. The envelope flap was raised a split-full-split approach in the coronal- apical direction. Flap mobilization was considered adequate when the marginal portion of the flap was able to passively reach a level coronal to the CEJ at each single tooth in the surgical site 2 mm. The root surface (only that portion of the root exposure) was mechanically treated with the use of curettes (Fig. 1B).
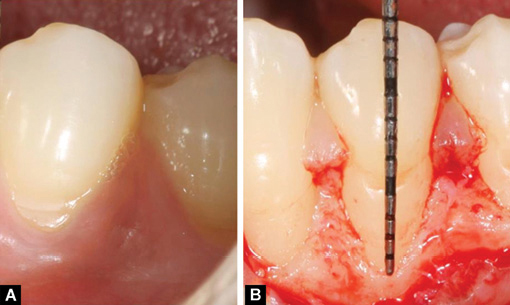
Fig. 1A and B: (A) Control site before surgery; (B) A buccal flap was elevated following root preparation. Note the deep bone dehiscence at tooth # 35
After the measurements at the recipient site were taken to know the dimensions of the graft, a modified Orban and Kirkland knife was used indistinctly to begin to remove the layer of epithelial tissue in the donor site of the palate. In addition, large round surgical bur and 15c scalpel were used to de-epithelialize the palate. The de-epithelialized area extended from the distal aspect of the canine to the distal aspect of the first molar; the most coronal area was 2 mm above the gingival margin and 5 mm from the mid-palatal suture medial border (Fig. 2A and B). The de-epithelialization at the palate donor site was continued until uniform bleeding was achieved indicating elimination of epithelium and exposure of the connective tissue. When prominent rugae were present, a more aggressive de-epithelialization procedure was used to flatten the rugae (Fig. 2C and D). The CTG of approximately 1–1.5 mm thickness was removed with the surgical blade (Fig. 3A). The donor site was compressed with a wet gauze for 2–3 minutes and sling sutures with Vicryl (4-0) (Ethicon, Hamburg, Germany) were used to control postoperative bleeding. After the flap was elevated, DeCTG was secured in position with interrupted resorbable sutures (Fig. 3B). The overlying flap was then coronally advanced over the donor tissue covering the latter as much as possible. The flap was secured in place with 5-0 PTFE monofilament sutures (Cytoplast, Osteogenics Biomedical Inc., Lubbock, Texas, USA) (Fig. 3C). Complete root coverage was observed 7 months after surgery in the control site (Fig. 3D). Test group: the same surgical protocol that was used in the elevation of the flap in the control was performed in the test group (Fig. 4). Seven months after surgery significant improvement in the degree of root coverage were observed in the test site (Fig. 5A and B). The PRF was obtained according to Choukroun et al.14 On the day of the surgery, the blood sample was drawn from a vein of the forearm of the patient and distributed in four sterile 10 mL tubes, which were centrifuged at 2,700 rpm for 12 minutes placing the same amount of blood in each tube (10 mL)and always facing each other to balance the weight and that the centrifugal forces are equal in a centrifuge with eight tubes (VLAD CF. 100VL; Sofia, Forlì-Cesena, Italy). This procedure was carried out in a simple manner in the same surgical moment (Fig. 5C and D).

Fig. 2A to D: (A) Occlusal view; (B) Palatal donor site de-epithelialized; (C) Harvesting of the connective tissue graft; (D) Donor site after de-epithelization

Fig. 3A to D: (A) Width of connective tissue graft; (B) Connective tissue graft placed; (C) Flap was coronally advanced and sutured with 5-0 expanded polytetrafluoroethylene; (D) Results after 7 months of surgery
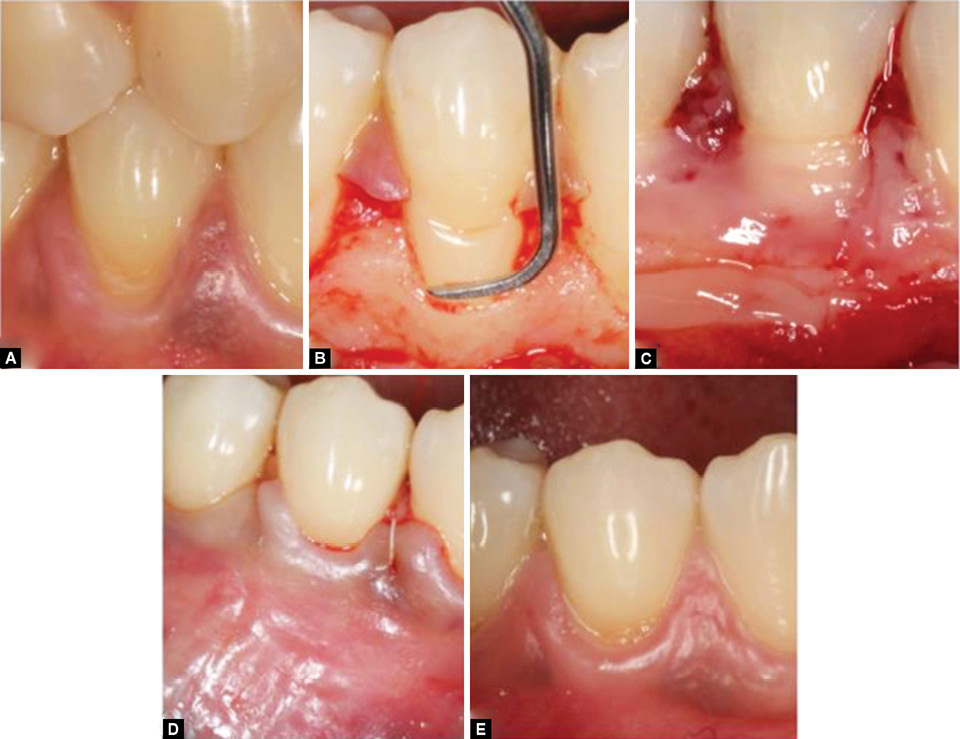
Fig. 4A to E: (A) Test site before surgery; (B) Flap elevation and root preparation; (C) PRF membrane being applied to the root surface; (D) Flap was coronally advanced and sutured with 5-0 expanded polytetrafluoroethylene suture material; (E) 7 months after surgery

Fig. 5A to D: (A) Initial view of test site before surgery; (B) 7 months after surgery; (C) Platelet-rich fibrin (PRF) clot tube; (D) PRF membrane
Postoperative Instructions and Follow-up
The postoperative instructions included 1 gm of amoxicillin plus clavulanic acid twice a day for 5 days, 25 mg of dexketoprofen for 2 days, and local irrigation of chlorhexidine 0.12%, three times a day for 14 days. Patients were informed to avoid brushing at the surgical site for 2 weeks, until suture removal, and to consume a liquid diet for 1 week and then a soft diet for 1 week more. Sutures were removed after 14 days, motivation and reinforcement of oral higiene and mechanical plaque removal when necessary were performed. Gingival recession (GR), PD, clinical attachment level (CAL), and GT were evaluated at baseline, 6 weeks, and after 28 weeks. Also, root coverage was measured at baseline and after 28 weeks.
Data Collection
At baseline, Löe and Silness gingival index and O’Leary plaque index were recorded. GR was measured from the CEJ to the gingival margin at the mid-buccal point of the teeth involved, using a periodontal probe (PCP-UNC 15 periodontal probe, Hu-Friedy, Chicago, Illinois). The gingival phenotype was categorized as either thin or thick according to the visibility of the underlying periodontal probe through the gingival tissue (visible = thin, not visible = thick).15
Statistical Analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) software version 17 (IBM® Company, Armonk, New York, USA) and STATA software Version 12 (Stata Corp., College Station, Texas, USA). Descriptive measures were estimated through frequency ratios for variables at 95% confidence intervals and 5% marginal error. Independent measurements for quantitative variables were compared using unpaired t-test or Mann–Whitney test, whereas dependent measurements were compared using paired t-test or Wilcoxon’s test according to data distribution. Comparisons of categorical variables between baseline and post-treatment were performed using McNemar’s test. p <0.05 was considered statistically significant in the current study.
RESULTS
Ten patients completed all examinations throughout 7 months. GI and PI scores were 0.24 ± 0.07 mm (mean: 0.23; min. 0.15, max. 0.40) and 0.85 ± 0.14 mm (mean: 0.86; min. 0.65, max. 1.06), respectively, in both study groups at baseline (Table 1). After surgeries, no serious morbidities were seen in either group and the healing went on normally. There were no statistically significant differences between the recession-type defects in the two groups at baseline and 28 weeks. GR was reduced from 3.30 ± 1.25 to 0.10 ± 0.32 mm in the PRF group and from 3.00 ± 1.63 mm to 0.30 ± 0.48 mm in the DeCTG group at 6 weeks (p = 0.63). Change from baseline to 6 weeks, was not statistically difference (p = 0.44), in the control and test group. The change of GR from baseline to 7 months was −3.40 ± 1.35 mm and −3.20 ± 1.93 mm (p = 0.67), in the DeCTG and PRF groups, respectively (Table 2).
| Parameter | Descriptive statistics |
|---|---|
| Mean ± SD Median (Min, Max) N = 10 |
|
| Age | 45.6 ± 11.14 45.60 (30.00, 63.00) |
| Gender Male Female |
50.00% 50.00% |
| O’Leary plaque | 0.24 ± 0.07 0.23 (0.15, 0.40) |
| Loe and Silness index | 0.85 ± 0.14 0.86 (0.65, 1.06) |
| Parameter | Control group (mm) | Test group (mm) | Difference (mm) | p value* |
|---|---|---|---|---|
| Mean ± SD Median (Min, Max) n = 10 |
Mean ± SD Median (Min, Max) n = 10 |
Mean ± SD Median (Min, Max) |
||
| Baseline | 3.30 ± 1.25 3.50 (2.00, 5.00) |
3.00 ± 1.63 3.00 (1.00, 7.00) |
−0.30 ± 1.50 0.00 (−3.00, 2.00) |
0.67 |
| 6 weeks | 0.10 ± 0.32 0.00 (0.00, 1.00) |
0.30 ± 0.48 0.00 (0.00, 1.00) |
0.20 ± 0.63 0.00 (−1.00, 1.00) |
0.63 |
| 28 weeks | −0.10 ± 0.32 0.00 (−1.00, 0.00) |
−0.20 ± 0.42 0.00 (−1.00, 0.00) |
−0.10 ± 0.57 0.00 (−1.00, 1.00) |
0.99 |
| Change from baseline to 6 weeks | −3.2 ± 1.23−3.00 (−5.00, −2.00) |
−2.70 ± 1.49−2.50 (−6.00, −1.00) |
0.50 ± 1.51 0.00 (−1.00, 3.00) |
0.44 |
| Change from baseline to 28 weeks | −3.40 ± 1.35−3.50 (−2.00, −5.00) |
−3.20 ± 1.93−3.00 (−8.00, −1.00) |
0.20 ± 1.81 0.00 (−3.00, 3.00) |
0.67 |
Regarding gingival phenotype in both groups at baseline and 28 weeks revealed that 80% (8/10) of test group had thick phenotype, 80% (8/10) of control group had thick phenotype, and only 20% (4/20) of individuals had thin phenotype reflected in both maxillary teeth; these percentages remained the same at baseline and at 28 weeks. 70% (14/20) of the evaluated teeth were located in the upper maxilla; 30% (6/20) were in the mandibular teeth (Table 3).
| Parameter | Type | Test group | Control group |
|---|---|---|---|
| N (%) | N (%) | ||
| Baseline | Thick phenotype | 8 (80.00) |
8 (80.00) |
| Thin phenotype | 2 (20.00) |
2A (20.00) |
|
| 28 weeks | Thick phenotype | 8 (80.00) |
8 (80.00) |
| Thin phenotype | 2 (20.00) |
2 (20.00) |
According to the results obtained, it can be concluded that when comparing the effectiveness of CAF + PRF or CAF + DeCTG for achieving root coverage, both procedures were effective for treating localized gingival recessions Miller class I and II.
DISCUSSION
The objective of this study was to evaluate, the effectiveness of a CAF + PRF or CAF + DeCTG for the treatment of recessions Miller class I or II and its evaluation in a follow-up up to 7 months. This period of time as a reference is sufficient for the measurement of the results in the clinical investigations with CAF + PRF, according to Oncu et al.16 since it constitutes the optimal period for the stability of the gingival margin.
Autologous blood processing has undergone a positive evolution from the PRP to the current PRF and the recent PRF-A or advanced, first by the highest concentration of viable cells and second by the slower and gradual release, up to 10 days.17 This is the main reason for choosing the PRF in our study. In the various systematic reviews of periodontal plastic surgery, scarce are the works in relation to the use of PRP or PRF in root coverage, and even less, based on randomized and controlled trials. Miron et al.18 analyzed in 14 randomized clinical trials the application of PRF and the therapeutic management of recessions. The authors compared CAF, DLSBF, CAF + CTG, CAF + EMD, CAF + AM with CAF + PRF, DLSBF + PRF, and CAF + CTG + PRF. The average root coverage ranges between 56% and 92.7%, contrasted with the results of the present study that reaches 98%, well above the previous ones and coinciding in the reviews in which it implies similar advantages and comparisons with groups that use CTG. Five studies compared CAF + PRF with CAF + CTG and found no significant differences, like the present, and averaging 85.3% in root coverage. One study compared CAF + CTG vs CAF + CTG + PRF found a significant increase for the second approach using CTG and PRF, in Miller class I or II of 40 patients. In conclusion, the last systematic review revealed that the use of PRF provides a slight gain in root coverage when compared to CAF only but does not produce better results when compared to EMD or CTG. Due to the homogeneous results and the high success in CR (over 80%) does not allow to determine very precise indications. Therefore, more research would be needed to establish in which clinical cases we could choose the CTG or PRF. An alternative is to rely on the structure of “evidence-based decision-making,” which establishes among the therapeutic objectives the issue, for example, of morbidity, as a relevant criterion in the choice of surgical alternatives in periodontal plastic surgical procedures.3
In the present study, all surgical procedures (DeCTG and PRF) were performed simultaneously by one surgeon. Also, the de-epithelization was performed on the palate, allowing better uniformity and easy manipulation of the CTG. McLeod et al.10 introduced the DeCTG technique together with a tunnel surgical procedure to treat multiple areas of gingival recession. The author showed that DeCTG is practical and simple to perform and that the partial palatal de-epithelialization resulted in a successful clinical outcome with increased gingival tissue thickness, keratinized tissue width, and root coverage.
In this study, the phenotype thickness was maintained at baseline and 28 weeks after surgery, in the study group, as well as in the control group. It is important to mention that both groups presented 80% of thick phenotype at the baseline. Therefore, it could be hypothesized that the presence of thicker tissue before surgery may explain the similar results obtained in both groups. The gingival phenotype thickness is an important factor for the maintenance of gingival health and the prevention of the progression of GR and PD. These gingival characteristics play a preponderant role in several clinical conditions of sites with gingival recessions, which allow it to have a positive therapeutic predictive value. Pini Prato et al.19 observed a recurrence in sites treated with only CAFs in a period between 6 months and 5 years of follow-up and was attributed to the smaller thickness and/or width of keratinized tissues. Therefore, the increase or maintenance of the gingival morphotype is a factor that will represent greater long-term tissue stability.
In a study conducted by Cheung et al.20 17 arches in 15 patients with bilateral gingival recessions were treated with SCTG and PRF covered by CAF. No statistically significant differences between the treatments at 8 months postsurgery, were found for complete coverage, VRD, CAL, and KTW. In contrast, Padma et al.21 investigated the additional benefits of PRF when used along with CAF. This study concluded that PRF membrane with CAF provides superior root coverage with additional benefits of gain in CAL and WKG at 6 months postoperatively. Also, Aroca et al.22 showed an increase in GT in the modified CAF + PRF group.
The limitations of this study are the relatively short time of follow-up; therefore, other studies can be necessary. Finally, the authors suggested to include in future research different methods, which may influence or improve the time of preparation, biomolecular components, such as growth factors and cytokines in order to close the gap of knowledge between biological and clinical response.
CONCLUSION
The present study indicated that both CAF + PRF and CAF + DeCTG techniques are effective procedures in the treatment of localized gingival recessions, and no significant differences were found in the percentages of root coverage.
ORCID
James R Collins https://orcid.org/0000-0001-7448-2784
REFERENCES
1. American Academy of Periodontology. Glossary of periodontal terms. 4th ed. Chicago, IL; 2001.
2. Zucchelli G, Mounssif I. Periodontal plastic surgery. Periodontol 2000 2015;68(1):333–368. DOI: 10.1111/prd.12059. PMID: 25867992.
3. Cortellini P, Pini Prato G. Coronally advanced flap and combination therapy for root coverage. Clinical strategies based on scientific evidence and clinical experience. Periodontol 2000 2012;59(1):158–184. DOI: 10.1111/j.1600-0757.2011.00434.x.
4. Sullivan HC, Atkins JH. Free autogenous gingival grafts. 3. Utilization of grafts in the treatment of gingival recession. Periodontics. 1968 Aug;6(4):152-60. PMID: 5243142.
5. Tenenbaum H, Klewansky P, Roth JJ. Clinical evaluation of gingival recession treated by coronally repositioned flap technique. J Periodontol 1980;51(12):686–690. DOI: 10.1902/jop.1980.51.12.686.
6. Langer B, Langer L. Subepithelial connective tissue graft technique for root coverage. J Periodontol 1985;56(12):715–720. DOI: 10.1902/jop.1985.56.12.715.
7. Roccuzzo M, Lungo M, Corrente G, et al. Comparative study of a bioresorbable and a non-resorbable membrane in the treatment of human buccal gingival recessions. J Periodontol 1996;67(1):7–14. DOI: 10.1902/jop.1996.67.1.7.
8. Pini Prato G, Tinti C, Vincenzi G, et al. Guided tissue regeneration versus mucogingival surgery in the treatment of human buccal gingival recession. J Periodontol 1992;63(11):919–928. DOI: 10.1902/jop.1992.63.11.919.
9. Rasperini G, Silvestri M, Schenk RK, Nevins ML. Clinical and histologic evaluation of human gingival recession treated with a subepithelial connective tissue graft and enamel matrix derivative (Emdogain): a case report. Int J Periodontics Restorative Dent. 2000 Jun;20(3):269-75. PMID: 11203568.
10. McLeod DE, Reyes E, Branch-Mays G. Treatment of multiple areas of gingival recession using a simple harvesting technique for autogenous connective tissue graft. J Periodontol 2009;80(10):1680–1687. DOI: 10.1902/jop.2009.090187.
11. Chambrone L, Sukekava F, Araújo MG, et al. Root-coverage procedures for the treatment of localized recession-type defects: a Cochrane systematic review. J Periodontol 2010;81(4):452–478. DOI: 10.1902/jop.2010.090540.
12. Tatakis DN, Chambrone L, Allen EP, et al. Periodontal soft tissue root coverage procedures: a consensus report from the AAP Regeneration Workshop. J Periodontol 2015;86(Suppl.):52–55. DOI: 10.1902/jop.2015.140376.
13. Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85(6):638–646. DOI: 10.1016/s1079-2104(98)90029-4.
14. Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF) Second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oran Pathol Oral Radiol Endod 2006;101(3):e56–e60. DOI: 10.1016/j.tripleo.2005.07.011.
15. Kan JY, Rungcharassaeng K, Umezu K, et al. Dimensions of peri‐implant mucosa: an evaluation of maxillary anterior single implants in humans. J Periodontol 2003;74(4):557–562. DOI: 10.1902/jop.2003.74.4.557.
16. Öncü E. The use of platelet-rich fibrin versus subepithelial connective tissue graft in treatment of multiple gingival recessions: a randomized clinical trial. Int J Periodontics Restorative Dent 2017;37(2):265–271. DOI: 10.11607/prd.2741.
17. Kobayashi E, Flückiger L, Fujioka-Kobayashi, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Invest 2016;20(9):2353–2360. DOI: 10.1007/s00784-016-1719-1.
18. Miron RJ, Zucchelli G, Pikos MA, et al. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig 2017;21(6):1913–1927. DOI: 10.1007/s00784-017-2133-z.
19. Pini Prato GP, Cairo F, Nieri M, et al. Coronally advanced flap versus connective tissue graft in the treatment of multiple gingival recessions: a split-mouth study with a 5-year follow-up. J Clin Periodontol 2010;37(7):644–650. DOI: 10.1111/j.1600-051X.2010.01559.x.
20. Cheung WS, Griffin TJ. A comparative study of root coverage with connective tissue and platelet concentrate grafts: 8-month results. J Periodontol 2004;75(12):1678–1687. DOI: 10.1902/jop.2004.75.12.1678.
21. Padma R, Shilpa A, Kumar PA, et al. A split mouth randomized controlled study to evaluate the adjunctive effect of platelet-rich fibrin to coronally advanced flap in Miller’s class I and II recession defects. J Ind Soc Periodontol 2013;17(5):631–636. DOI: 10.4103/0972-124X.119281.
22. Aroca S, Keglevich T, Barbieri B, et al. Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: a 6-month study. J Periodontol 2009;80(2):244–252. DOI: 10.1902/jop.2009.080253.
________________________
© The Author(s). 2021 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.