EDITORIAL | https://doi.org/10.5005/jp-journals-10024-3213 |
Autophagy: The “Pac-Man” within Us—Ally or Adversary?
1Faculty of Dentistry, SEGi University, Petaling Jaya, Selangor, Malaysia
2Department of Prosthodontics and Restorative Dentistry, School of Dentistry, College of Medicine and Health Sciences, University of Rwanda, Remera Campus, Kigali, Rwanda
3Department of Oral Maxillofacial Clinical Sciences, Faculty of Dentistry, University of Malaya, Kuala Lumpur, Malaysia; Oral Cancer Research and Coordinating Centre, Faculty of Dentistry, University of Malaya, Kuala Lumpur, Malaysia
4Department of Oral Pathology, Faculty of Dental Sciences, MS Ramaiah University of Applied Sciences, Bengaluru, Karnataka, India
5Department of Maxillofacial Surgery and Diagnostic Science, Division of Oral Pathology, College of Dentistry, Jazan University, Jazan, Saudi Arabia
Corresponding Author: Anitha K Pandarathodiyil, Faculty of Dentistry, SEGi University, Petaling Jaya, Selangor, Malaysia, e-mail: anithakrishnan@segi.edu.my
How to cite this article: Pandarathodiyil AK, Vijayan SP, Ramanathan A, et al. Autophagy: The “Pac-Man” within Us—Ally or Adversary? J Contemp Dent Pract 2021;22(10):1079–1081.
Source of support: Nil
Conflict of interest: None
Autophagy, first described by the Belgian biochemist Christian De Duve in 1963, is derived from the Greek word “autóphagos,” which means “self-devouring.” It is a cellular homeostatic process in which the body rids itself of flawed or damaged cells and other defective cellular organelles.1 The implication of defective autophagy in various human diseases has been well documented. The vital importance of autophagy is underscored by the fact that robust cellular health and function are intricately linked with it.
Four varying types of autophagy have been studied but among them, macroautophagy assumes particular significance. The significance is that if certain key cellular components like mitochondria become defective or obsolete in function, then the defective component is identified and detached from the remaining contents of the cell through a two-pronged membrane vesicle called an autophagosome.2–4 Subsequently, this vesicle fuses with an available lysosome, which is a digestive enzyme chiefly responsible for the cellular breakdown, and thus, the defective or degraded parts of a cell are eliminated with efficiency and precision. Eventually, this coalesced vesicle, now referred to as an autolysosome, undergoes degeneration and the ensuing content is reprocessed (Fig. 1).
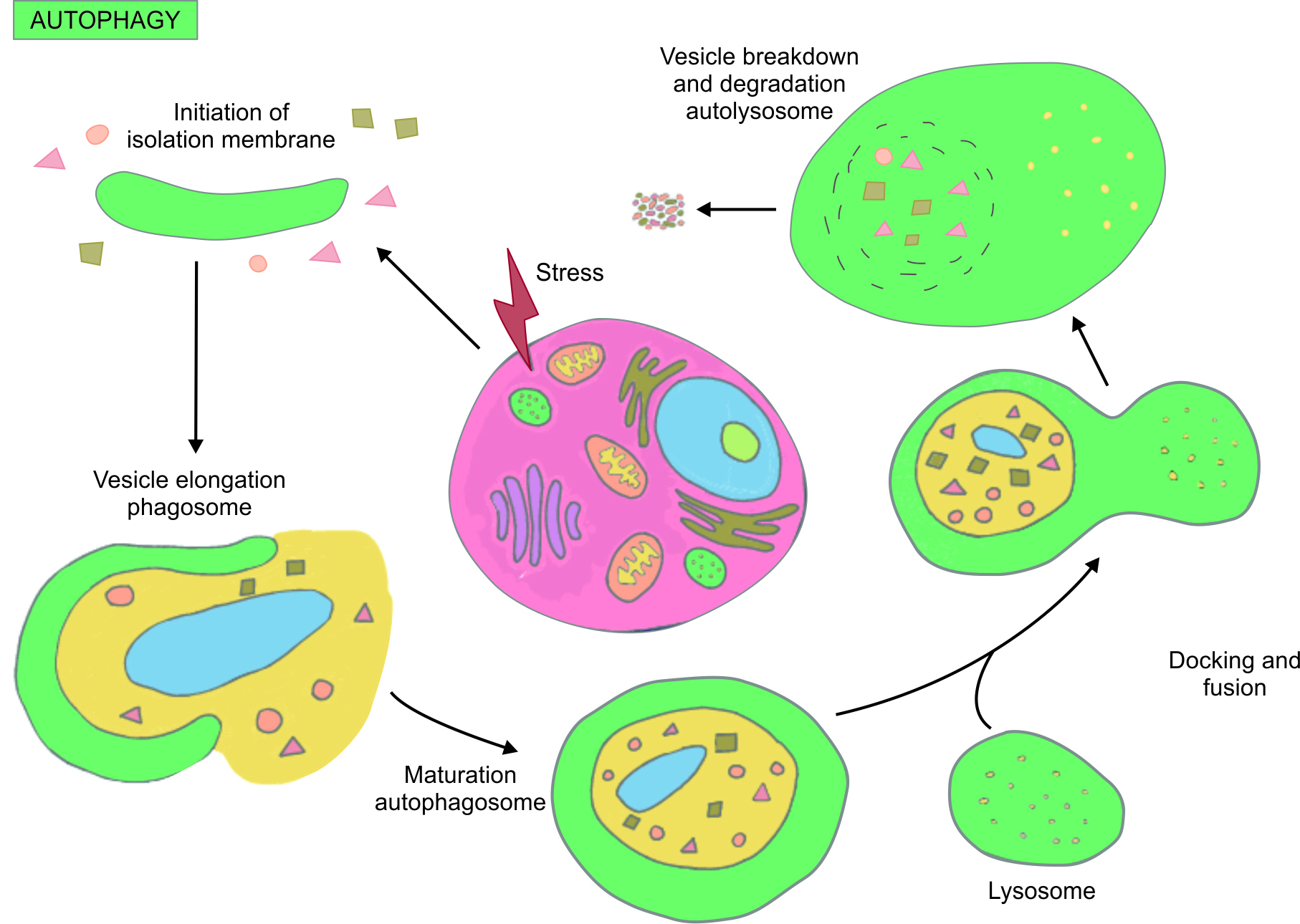
Fig. 1: The autophagy pathway
Hence, the initial widely upheld assumption that autophagy is a highly efficient and effective response elicitor against stress and accumulated toxins has subsequently been well elucidated and validated.5 In contemporary times, the attention devoted by the scientific community to further advance our comprehension of this complex but pivotal evolutionary process can be attested by the fact that Yoshinori Ohsumi, a renowned Japanese cell biology professor, was awarded the Nobel Prize in medicine (2016) for his stellar, ground-breaking work on the mechanisms of autophagy.
Natural inducement of autophagy is the norm but under some extraordinary circumstances, deviation from the norm can occur. For example, fasting or the usage of specialized/ketogenic diets can induce autophagy as nutrient depletion can engender an acute autophagic reaction. During fasting, perceptible changes in the metabolic processes occur inevitably as cell survival becomes paramount and autophagic influence kicks in to maintain the status quo on cellular viability and energy.6,7 Recent studies undertaken to examine the autophagic response to bacterial infection have adequately demonstrated that some of the bacterial pathogens can escape the conventional lysosomal trap by reconfiguring and modifying themselves, thereby unwillingly associating themselves into the host cytoplasm.8 These transfigured pathogens are subsequently degraded and disintegrated by autophagosomes.9
Nonetheless, in a classic role reversal, certain bacterial pathogens (e.g., Porphyromonas gingivalis) undergo a cellular transformation, which instead of culminating in phagocytosis, transforms the pathogens into a transmogrified structure that resists degradation and destruction.10 Further study and research on the effect of autophagy on viral pathogens have exhibited similar outcomes. This alludes to the fact that apart from the control of viral virulence by the traditional destructive pathways, these pathogens employ distinct, unique blueprints in attempting to refine and improve autophagy gesturing indicating, to achieve two important objectives: (i) avoid obliteration and (ii) exploit the significant functional advantages given by typical autophagy action.11
A ground-breaking study carried out to examine autophagy activity on patients suffering from pulmonary diseases and compare them with a control group resulted in markedly increased autophagy activity on the lung tissues of the patients suffering from chronic obstructive pulmonary disease and emphysema while affirming that early growth response-1 is the vital mediator gene in orchestrating this response but interestingly, no palpable increase in autophagy activity was discerned on patients suffering from other pulmonary disorders.12
Nevertheless, the present knowledge with respect to the effect of the autophagic process on certain pathophysiological conditions and human diseases mirrors our token understanding of the autophagic process on human tumor. The apparent role of autophagy in tumor suppression/development is complicated. Conventional scientific knowledge points to the fact that the “self-cleansing process of autophagy” should be able to effectively vanquish tumor growth by eliminating damaged and defective cells and cellular organelles.13 However, in reality, it is not as simple as it seems. Strange as it may appear, recent studies have shown us that where there are extensive evolution of neoplastic processes and malignant transformation of cells, autophagy has been seen to be intricately associated with tumor maintenance and promotion.14,15
Thus, the role reversal of autophagy as the tumor advances draws attention to the fact that autophagy can be construed as a mixed blessing. This is because as tumor cells evolve and degenerate further, autophagy plays a vital role in keeping the tumor viable by effectively eliminating the overtly weak and defective cells and cellular organelles without being able to expunge the malignant process that is already set in motion.16,17
Recent scientific studies with regard to the effect of autophagy on cancers, particularly oral cancers, have thrown up intriguing results, thereby validating the urgent need for more focused and robust research to harness potential benefits.18,19 Inhibition of autophagy and thereby inducing apoptosis by appropriate agents may be a therapeutic measure in certain drug-resistant cancers, thus exposing the dark side of autophagy.19 However, as a note of caution, any discernible and eager anticipation in leveraging the apparent advantages of the autophagy process has to be tempered with the sobering reality of existing scientific knowledge.
However, on a more optimistic note, wide scientific acknowledgment of the intensely monitored and numerous signaling pathways as an established reaction to a wide variety of autophagy stimuli for improving cellular health and vitality and rectifying organelle function has enabled us to hone our understanding of the considerable influence of autophagy on the physiological human aging process. Therefore, it can be affirmed that an imperiled autophagy process is synonymous with age-associated illnesses.20 As a result, discovery and recognition of pivotal genes responsible for the autophagy process can pave way for the reinstatement of normal autophagy pathways.
Recent scientific studies done on numerous organisms have been remarkably successful in isolating and determining these genes.21 It is now a fact that advancing age oversees a palpably frailer autophagy process and this valuable information can prove to be a game-changer in developing future effective treatment protocols for certain age-related diseases such as Huntington’s disease and Alzheimer’s disease.22,23 Going forward, more extensive research can go a long way in facilitating more fruitful investigations that may be undertaken with the intent of enhancing healthy living and augmenting cellular health.
To conclude, the myriad and fascinating knots tying autophagy with human disease and normal physiology are being slowly but painstakingly undone. However, key answers to certain elementary questions still elude the scientific community.24 The proverbial “Jekyll and Hyde” nature of autophagy effects on different stages of the same disease progression continues to confound us. While autophagy activity has been indistinguishably linked with several diseases, this has not yet translated into the evolution of effective treatment strategies since the chief disease-causing agent remains obscure. A formidable challenge awaits us but hope soars with newer, cutting-edge research that expedites scientific progress as we seek an imminent breakthrough to make our world a better and healthier place to dwell in.
ORCID
Anitha K Pandarathodiyil https://orcid.org/0000-0002-5436-0138
Srinivas P Vijayan https://orcid.org/0000-0003-2636-9861
Anand Ramanathan https://orcid.org/0000-0001-6496-7139
Shankargouda Patil https://orcid.org/0000-0001-7000-6609
REFERENCES
1. Levine B, Kroemer G. Autophagy in the pathogenesis of the disease. Cell 2008;132(1):27–42. DOI: 10.1016/j.cell.2007.12.018.
2. Klionsky DJ, editor. Autophagy. Georgetown, TX: Landes Bioscience; 2004. p. 1–303.
3. Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol 2007;9(10):1102–1109. DOI: 10.1038/ncb1007-1102.
4. Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 2011;27:107–132. DOI: 10.1146/annurev-cellbio-092910-154005.
5. Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell 2010;40(2):280–293. DOI: 10.1016/j.molcel.2010.09.023.
6. Antunes F, Erustes AG, Costa AJ, et al. Autophagy and intermittent fasting: the connection for cancer therapy? Clinics (Sao Paulo) 2008;73(Suppl. 1):e814s. DOI: 10.6061/clinics/2018/e814s.
7. McCarty MF, DiNicolantonio JJ, O’Keefe JH. Ketosis may promote brain macroautophagy by activating Sirt1 and hypoxia-inducible factor-1. Med Hypotheses 2015;85(5):631–639. DOI: 10.1016/j.mehy.2015.08.002.
8. Kirkegaard K, Taylor MP, Jackson WT. Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat Rev Microbiol 2004;2(4):301. DOI: 10.1038/nrmicro865.
9. Rich KA, Burkett C, Webster P. Cytoplasmic bacteria can be targets for autophagy. Cell Microbiol 2003;5(7):455. DOI: 10.1046/j.1462-5822.2003.00292.x.
10. Dorn BR, Dunn WA Jr. Progulske-Fox A. Bacterial interactions with the autophagic pathway. Cell Microbiol 2002;4(1):1. DOI: 10.1046/j.1462-5822.2002.00164.x.
11. Suhy DA, Giddings TH Jr. Kirkegaard K. Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy-like origin for virus-induced vesicles. J Virol 2000;74(19):8953. DOI: 10.1128/jvi.74.19.8953-8965.2000.
12. Chen ZH, Kim HP, Sciurba FC, et al. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One 2008;3(10):e3316. DOI: 10.1371/journal.pone.0003316.
13. Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene 2004;23(16):2891. DOI: 10.1038/sj.onc.1207521.
14. Ogier-Denis E, Codogno P. Autophagy: a barrier or an adaptive response to cancer. Biochim Biophys Acta 2003;1603(2):113. DOI: 10.1016/s0304-419x(03)00004-0.
15. Cuervo AM. Autophagy: in sickness and in health. Trends Cell Biol 2004;14(2):70. DOI: 10.1016/j.tcb.2003.12.002.
16. Paglin S, Hollister T, Delohery T, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res 2001;61(2):439. PMID: 11212227.
17. Alva AS, Gultekin SH, Baehrecke EH. Autophagy in human tumors: cell survival or death? Cell Death Differ 2004;11:1046. DOI: 10.1038/sj.cdd.4401445.
18. Son SH, Kim EJ. Autophagy and oral cancer. J Life Sci 2017;27(8):958–964. DOI: 10.5352/JLS.2017.27.8.958.
19. Patil S, Rao RS, Raj AT. Dual role of autophagy in oral cancer. J Int Oral Health 2015;7(6):i–ii. PMID: 26124615.
20. Martinez-Lopez N, Athonvarangkul D, Singh R. Autophagy and aging. Adv Exp Med Biol 2015;847:73–87. DOI: 10.1007/978-1-4939-2404-2_3.
21. Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science 2003;299(5611):1342. DOI: 10.1126/science.1077991.
22. Shibata M, Lu T, Furuya T, et al. Regulation of intracellular accumulation of mutant Huntingtin by Beclin 1. J Biol Chem 2006;281(20):14474–14485. DOI: 10.1074/jbc.M600364200.
23. Lipinski MM, Zheng B, Lu T, et al. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc Natl Acad Sci USA 2010;107(32):14164–14169. DOI: 10.1073/pnas.1009485107.
24. Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science 2004;306(5698):990–995. DOI: 10.1126/science.1099993.
________________________
© The Author(s). 2021 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.